Understanding Ammonia Refrigeration Systems
Understanding the differences among the three types of ammonia refrigeration systems and how each works is a necessity for every technician that works on them
Article by Chris Harmon and Rick Dumais
As far back as we can remember man has used ammonia for one reason or another. It is in fact the oldest known refrigerant. It is successfully utilized in many industries including food, petrochemical and pharmaceutical. So why is it so misunderstood?
In this article, we will attempt to clear up and demystify anhydrous ammonia, which is a formulation void of water, as it relates to refrigeration. We will discuss the benefits and drawbacks of this ammonia refrigerant as well as review leak detection and safe handling procedures.
Related service: Process Safety Management Program.
Why Ammonia?
Why do we use anhydrous ammonia instead of halocarbons in industrial applications? Ammonia is cheap and extremely efficient. Ammonia is the most commonly used refrigerant worldwide for large commercial applications. The main use of ammonia is agricultural (fertilizer). More than 80 percent of the ammonia produced is utilized in this fashion due to its high nitrogen content. Since ammonia is plentiful, the cost is low. The cost for refrigerant-grade anhydrous ammonia typically is less than 50 cents a pound, compared to about $7 a pound for R4O4A and $15 a pound for R-502.
There are two primary grades of ammonia commonly available in the marketplace. There is an agricultural or commercial-grade ammonia that must contain a minimum water content of at least 2,000 ppm water (0.2 percent) with a maximum water content of 5,000 ppm (0.5 percent). The minimum water content prevents stress corrosion cracking of the metals used in equipment for the agricultural industry.
Industrial-grade anhydrous ammonia, commonly called metallurgical or refrigeration grade, has very little water contamination. Metallurgical-grade has a maximum of about 33 ppm water (0.0033 percent) and refrigeration-grade has a maximum of about 150 ppm water (0.015 percent). For optimum efficiency and effectiveness in your industrial refrigeration system, the ammonia supplied to you for your system should meet or exceed these specifications.
In addition to the price the most compelling reason to utilize anhydrous ammonia is the fact that it has such a high latent capability per pound. Its latent capability at 5° F evaporator temperature is 565 Btu per pound. When compared with R-22, which is approximately 69 Btu per pound at the same temperature, its obvious that it takes less ammonia to do the work because it is more efficient. This means less kwh used and lower operating costs.
Typical Systems
The best way to understand ammonia refrigeration sytlems is to review the basic designs used today. Some ammonia-based systems are similar to designs used in hvacr systems that use halocarbons. The differences are somewhat obvious but offer just enough differences to challenge technicians from either field.
We will discuss the benefits, drawbacks and special problems associated with each type of system. Three basic types of systems exist: thermal expansion valve (TXV), flooded and liquid recirculating (or liquid overfeed).
Thermal Expansion Valve (TXV) Systems
To ensure complete vaporization, a thermal expansion valve is used to control flow (see Figure 1). The refrigerant vapor is allowed to superheat about 10° F at the end of the evaporator coil. Although they are less efficient, the TXV systems are simpler in design, require less refrigerant charge, cost less initially and usually have less oil-logging problems.
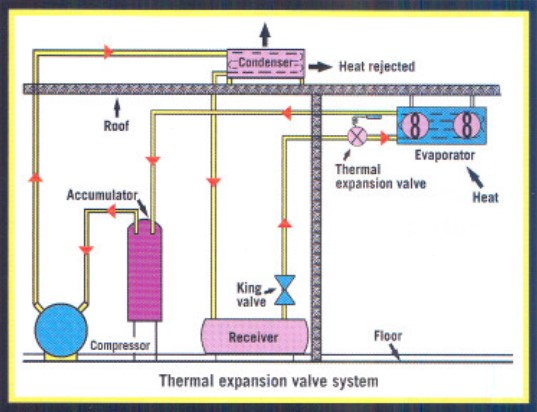
Many thermal expansion systems are in use today. They work well as long as there are minor load swings in the system.
Flooded Systems
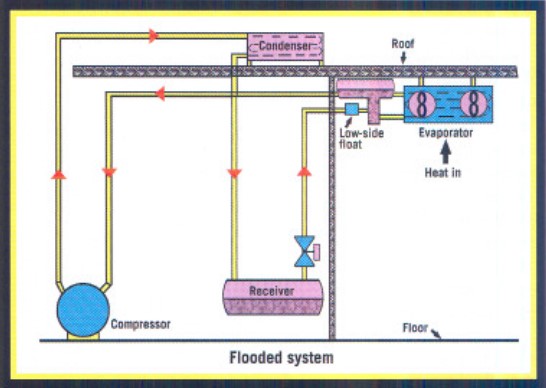
A typical flooded system consists of a surge drum with some type of liquid-level control device, generally a low-side float (see Figure 2). A liquid leg feeds the liquid to the bottom of the coils. As the liquid entrained with the gas is returned to the top of the surge drum, it falls into the leg and allows the gas to be drawn off the top into the suction of the ammonia compressor.
The system is designed to cause a boiling action that will result in about 2 to 3 pounds of liquid being recirculated for every pound of ammonia refrigerant evaporated. There is more refrigerant recirculated than vaporized.
Some advantages of a flooded system compared to a TXV system include:
- More effective use of evaporator surfaces.
- Easier distribution of refrigerant with parallel systems.
- Cooler vapor entering the ammonia compressor (which reduces the discharge temperature).
- Higher initial cost.
- More refrigerant is required in the system.
- Oil accumulation (logging) is more severe requiring closer monitoring.
Liquid Recirculation Systems
This wetted vapor is returned to a low-pressure receiver where separation of the liquid and gas occurs (see Figure 3). The overfeed system ensures that a constant liquid level is maintained in the accumulator (sometimes referred to as a low-pressure receiver).
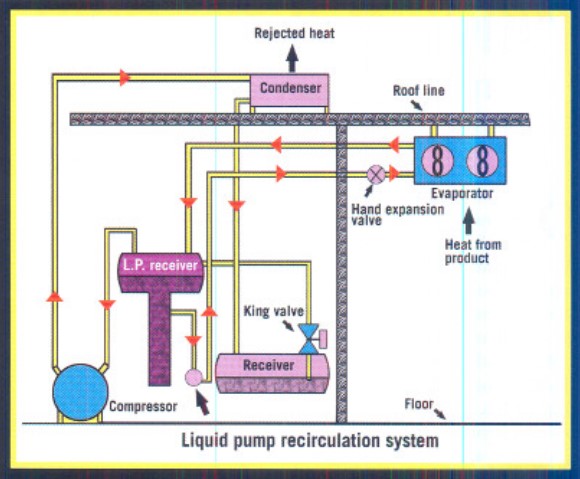
- High system efficiency.
- Efficient hot gas defrosting capability.
- Simplified oil draining and return.
- Lower coil temperatures.
The disadvantages include:
- Larger refrigerant charge is required.
- Larger piping is required.
The Safety Factor
Most serious cases of leaks and/or explosions can be attributed to accidents. (The U.S. Occupational Safety and Health Administration (OSHA) and the International Institute of Ammonia Refrigeration (AR) do not keep injury and fatality statistics for ammonia-based systems.)
One advantage of ammonia over halocarbon-based refrigerants is that it is self-alarming due to its strong and distinct odor. The following table lists some of the possible effects of ammonia in the air:
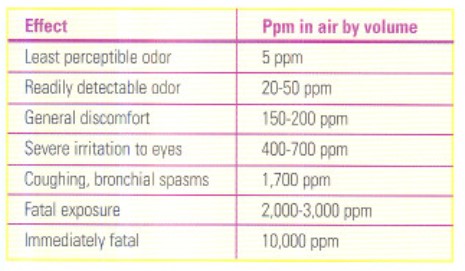
Because the smell of ammonia is readily perceptible, you will know it if there's even a tiny leak. With halocarbons a technician’s chances of smelling a leak are low, unless it contains a lot of oil and you are near the source.
Ammonia vapor is lighter than air and in a confined area il displaces oxygen from the ceiling downward. Halocarbons are heavier than air and will displace the oxygen from the floor upward. Either situation can be fatal.
Ammonia is flammable and has a lower explosive limit (LEL) of 15 percent (150,000 ppm) and an upper explosive limit (UEL) of 28 percent (280,000 ppm). When the ammonia vapor is mixed with a mistable oil, the LEL can be as low as 8 percent (80,000 ppm).
Ammonia also will undergo what is called hazardous decomposition at temperatures above 850° F. This means it will break down into nitrogen and hydrogen gases. Hydrogen gas has a flammability range of 4 to 75 percent.
Ammonia systems generally are built around an understanding of the characteristics of anhydrous ammonia and its dangers and benefits. As with any system, the first line
of defense is the safety engineering designed into it.
Industry safety standards continue to be the main reason ammonia systems are much safer than most people are aware. Each system must meet strict safety codes, which include materials of construction as well as appropriate relief valves, ventilation, safety switches and other safety engineering concerns.
Related service: Valve Tagging and Labeling.
Training And Safety
As in any other industry, the training of operators and technicians is important. Fully trained operators and technicians are much less likely to cause a situation resulting in death or injury.
Both OSHA and U.S. EPA address training for larger ammonia refrigeration systems. If the ammonia refrigeration system has a charge of 10,000 pounds or more, you must implement or take operational and maintenance training. Many companies follow this regardless of the charge level.
Training must include standard operating procedures for every task that will be performed, as well as safe work practices, including proper line-break procedures. Line-break procedures are what a technician does each time the ammonia refrigeration system is opened for maintenance. Additionally, operating personnel must receive refresher training at least every three years.
Related service: Level I Refrigeration Operator.
Finding Ammonia System Leaks
The second part of a series on ammonia refrigeration systems explores the various tools and sensors that are available to help service techs find system leaks
Most modern ammonia systems are computer controlled in one manner or another. They require an initial setup which consists of the basic programming of the operating system. This is when a system's operational parameters and set-points are established.
A system's performance is dictated by the set-points burned into the software that controls the normal operation of the compressors, condensers, fans, evaporators and other essential components. Many older systems are either manually operated or partially computerized, which creates a different set of operational tasks.
As with any industrial refrigeration system, safety devices and controls are essential to assure safe operation under normal and abnormal conditions. Many different devices are used to perform different tasks during the course of a normal operating day.
Float switches have been used in our industry for many years, and although they have become more sophisticated they still perform the same basic functions. Many are used to control ammonia refrigerant levels as well as control on and off functions of solenoid valves and regulators.
Several other devices function as safety switches, valves, pressure regulators and electrical interlock devices. The number of devices is so large that it is beyond the scope of this article to detail all of them.
Finding Leaks
Anhydrous ammonia is a Class I refrigerant and is an inhalation irritant as well as a caustic. This can create a problem for most of us because humans cannot tolerate exposure to large amounts of this refrigerant very well.
Levels of concentration and resulting protective actions taken will varv. At a low concentration level of 0 to 25 parts per million (ppm), no respirator is required and a person can work for eight hours without any adverse effects. At a concentration level of 300 ppm a full-face air purifying respirator must be worn.
Above 300 ppm a self-contained breathing apparatus (SCBA) must be worn. Additionally, a fully encapsulated suit must be worn when the concentration level is injurious to the skin. This level begins at around 300 to 500 ppm. It is common to put on the suit when the SCBA is worn.

Calibrate these devices on a regular basis, The most popular of them utilizes an electrochemical sensor. The sensor contains a cell whose life is dependent upon the amount of ammonia it is exposed to as well as air temperatures it is subjected to, and must be replaced every two years, These sensors also must be replaced when they can no longer be calibrated.
Automated functions of these devices include turning on exhaust fans, turning off liquid feed solenoids or even shutting down the entire refrigeration system. An alarm system is just one of many tools that will ensure that the system is operating as safely as possible.
An ‘Alarming’ Condition
The unit, which is permanently mounted on a wall, allows the operator to adjust the concentration range (ppm) to reach before sending a signal. This is useful as the placement of the unit may require a less sensitive response due to possible higher concentration levels during maintenance tasks. This fixed-type of sensor has been used for many years and comes in many different designs.
Meeting the need for smaller, more portable units has proven a challenge as the level of detection and the durability factors come into play. Above are a few of the portable sensing devices used for sampling the air for ammonia to help find leaks or to determine how much ammonia is present.
Popular handheld units can work well to detect levels up to 500 ppm and are easy to operate. For smaller leaks, the unit shown at the top right of this page works well.


The unit pictured is a draw-type bellows sampler, which draws a measured amount of air into the bellows through the glass tube. The results show up on the glass tube as a color change, which intensifies as the concentration increases, This type of sampler works well as a tool for first responders entering a suspected leak area or “hot zone.”
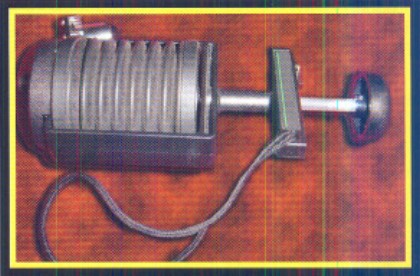
If portability, instant readings and higher concentration readings are required, such as with hazardous material response teams, the unit shown at the top of this page will sample leaks up to 20,000 ppm. The unit has an extendible wand to measure the levels in the surrounding area. It is designed for use by first responders.

It does require some skill and experience to detect and isolate a leak in a large area. The smoke can get quite heavy if the leak is large enough to quickly fill the room with smoke. Some of us would rather smell ammonia than a sulfur stick as it has a strong and lasting odor when burning.
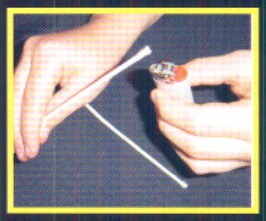
The use of these tools requires training by qualified professionals who know and understand ammonia and its characteristics. There is no substitute for formal training - safety always begins in the classroom.
A good refrigeration system starts with good engineering and a proven design. Safety engineering is the first line of defense for these systems. Then comes proper training of technicians.
Next month's article will address specific problems related to an ammonia refrigeration system, including fixing leaks and repairing components.
How to Detect and Fix Ammonia System Leaks
Part three of a series on ammonia refrigeration systems reviews the tools needed to detect system leaks and how to fix them
But there are several other ways to detect a leak varying from something as simple as a soap test or as sophisticated as an electrochemical sensing device. (See the sidebar on page 7 for details on the best leak-detection tools and methods to use and when.)
Finding a leak with pressures above atmospheric pressure (greater than 14.7 psia or 0 psig) is somewhat easier because the refrigerant is leaking out of the system into the air. On systems or portions of them with pressures below atmospheric pressure (less than 14.7 psia or 0 psig typically measured in inches of mercury) it is much more difficult because air is being drawn into the refrigeration system.
Leaks can occur in almost any part of an ammonia refrigeration system or most other industrial refrigeration systems for that matter. The following pictures show areas where leaks commonly are found and how to fix each leak:
Bonnets And Stem Packing
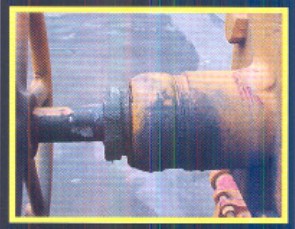
This type of valve arrangement also can have bonnet and/or flange problems and require pumpdown, and a gasket or seal replacement to fix the leak. The repair of this leak would require evacuation of the valve area and possibly the connecting pipe to isolate the valve. Then the valve could be serviced and placed back into operation,
Sight Glasses

Close the top isolation valve for the column and the remaining vapor will release into a bucket of water. Unscrew the glass and install a new O-ring. This job could take two to four hours depending on the situation.
Seals Around Screw Compressors
Fixing a leak on the screw compressor shown above is a fairly simple replacement job that still requires some expertise. Managers of some facilities either have a manufacturer's representative replace the seal on-site as warranty work or sometimes go as far as dismounting and sending the unit back to the manufacturer for repair and re-sealing.
If the technician and/or operator is qualified, do the replacement in-house, Most manufacturers supply tshe special tools required to replace the seal, should it leak, with the purchase of the compressor. Simply follow all manufacturer instructions on the replacement job.
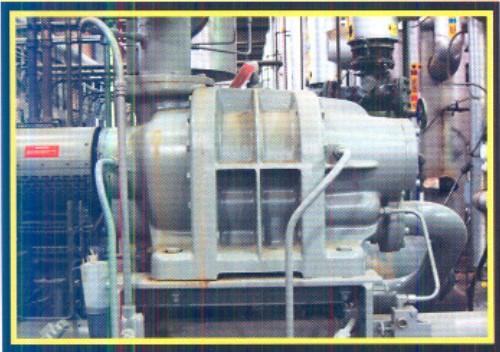
Seals Around Liquid Ammonia Pumps
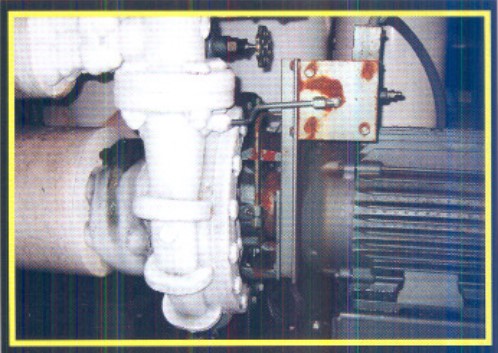
This repair requires some expertise in the ammonia pump's design and knowledge of the manufacturer's requirements. Qualified techs or operators need to reseal most of these pumps in the field. The job could take two to four hours with only a few special tools being required.
Around Back-Pressure Regulators (BPRs)
Use the unit's cut sheet, which should have accompanied the regulator when it was purchased, or a copy of it to make sure the proper regulator parts are used, as many of these look alike in color and design. Alter the regulator is placed back into service, make a pressure adjustment to assure that the regulator is operating within the set points for the refrigeration system to which it is connected.
Leaks on the portions of the ammonia refrigeration system that are below atmospheric pressure can be difficult to find. The only type of detector that can help an operator locate a vacuum leak is an ultrasonic leak detector. This detector finds the leaks by the noise created when air is drawn into the system,
Sometimes you can pressure up the system. Most leaks that occur under vacuum, however, have a tendency to seal when placed under positive pressure.
A Variety of Leak Detection Tools, Methods are Available
Soap Solutions
A variety of soap solutions are commercially available. The soap solution is applied to a suspected leak. Ifa leak exists, bubbles will form indicating the exact location.
Litmus Paper
The paper first must be dampened or wetted slightly and then placed around the suspected leak area. When ammonia refrigerant is encountered the paper will change color. The color can range from a light pink to a bright red depending on the severity of the leaks.

Most technicians and/or operators wet the paper with their tongues but you also can use water from a container. The paper is not an effective way to locate larger leaks because it turns completely red and usually only verifies what your eyes and nose already have told you: A large leak is evident.
Sulfur Stick
As with litmus paper, using sulfur sticks to find large leaks tends only to confirm that a leak exists. The problem with a sulfur stick is that with a large leak you simply end up smoking up the area close to the leak source. But for small-to-medium leaks they work fine as long as the person using them is familiar with the methods used to find the exact leak location.
A small-to-medium-sized leak usually is easy to find if it is in an open area. The smell and a visual display are great indicators. But not all leaks are within reach or in the open. The proper use of a sulfur stick can help locate leaks that are hidden from view.
As illustrated in the picture (bottom, left) the leak is from the valve packing as indicated by the smoke trail. In most cases you can detect a leak this size by smell. The person using the sulfur stick first must set the end of the sulfur stick on fire or it will not produce the smoking effect.

This is fairly simple to perform if the valve in question is a back-seating type. If not, a more detailed procedure would involve pumping down the liquid line feeding the valve as well as isolating the downstream side of the valve.
As mentioned above, using a sulfur stick to pinpoint a leak's location can be difficult. Practice will make a technician more efficient at finding leaks. One of the drawbacks to using a sulfur stick is that most operators would rather smell ammonia than the sulfur stick. The stick has a strong, lingering odor even after the testing is complete. It has a tendency to leave a bad taste in your mouth for several days.
Caution is urged when using a sulfur stick because ammonia is flammable. Pockets of ammonia can ignite or flash, creating a potential hazard. Be cautious when there is poor air circulation that can cause ammonia to build up. Properly used, sulfur sticks are an effective tool for finding ammonia leaks.
Electrochemical Detectors

Electrochemical detectors have a limited range, typically either 0-300 ppm or 0-1,000 ppm. These types of detectors are sensitive and can find leaks that cannot be located with sulfur sticks or litmus paper. — Richard M. Dumais and Chris Harmon
Richard Dumais is president of the Ammonia Refrigeration Technicians Association (ARTA). He also is an active ammonia refrigeration instructor and and experienced refrigeration systems operator. Chris Harmon is a managing partner for Industrial Consultants LLC (www.ammonia.com) and has 25 years of experience in training, consulting, design, engineering and operations. Visit ARTA at www.nh3tech.org for more information)
Reprinted From RSES Journal

